Answer:
The final pressure in the chamber is 5 atm.
Step-by-step explanation:
The balanced chemical reaction is:
2 N₂ (g) + O₂ (g) ⇒ 2 N₂O (g)
Initially, we have 4.00 atm of N₂ gas and 3.00 atm of O₂ gas. These are the partial pressures of each gas.
Nitrogen is the limiting reactant because it gives the fewest moles of reaction.
The initial state of the reaction is: 4.00 atm of N₂ gas and 3.00 atm of O₂
During the reaction, 4 atm of N₂ and 2 atm of O₂ are consumed to form 4 atm of N₂O
Finally, 1 atm of O₂ and 4 atm of N₂O remain
![\left[\begin{array}{cccc}&2N_2&O_2&2N_2O\\I:&4&3&-\\R:&-4&-2&+4\\F:&0&1&4\end{array}\right]](https://img.qammunity.org/2020/formulas/chemistry/college/74khkoyhxe0pos57c1w9pj84jqzsn4xpv9.png)
Total Pressure of the chamber:
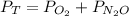 = 1 atm + 4 atm=5 atm
= 1 atm + 4 atm=5 atm
The final pressure in the chamber is 5 atm.