Answer: high temperature and low pressure
Step-by-step explanation:
The Ideal Gas equation is:
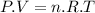
Where:
 is the pressure of the gas
is the pressure of the gas
 is the volume of the gas
is the volume of the gas
 the number of moles of gas
the number of moles of gas
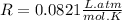 is the gas constant
is the gas constant
 is the absolute temperature of the gas in Kelvin
is the absolute temperature of the gas in Kelvin
According to this law, molecules in gaseous state do not exert any force among them (attraction or repulsion) and the volume of these molecules is small, therefore negligible in comparison with the volume of the container that contains them.
Now, real gases can behave approximately to an ideal gas, under the conditions described above and taking into account the following:
When temperature is high a real gas approximates to ideal gas, because the molecules move quickly, preventing the repulsion or attraction forces to take effect. In addition, at low pressures, the volume of molecules is negligible.