Answer: No, it is impossible
Step-by-step explanation:
When we talk about electrons being ejected from a metal, we are talking about the photoelectric effect, which consists of the emission of electrons (electric current) that occurs when light falls on a metal surface under certain conditions.
This is what Einstein proposed with the photoelectric effect:
Light behaves like a stream of particles called photons with an energy
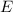 , which has an inverse relation with the wavelength
, which has an inverse relation with the wavelength
 (this means the smaller
(this means the smaller
 is the higher the energy):
is the higher the energy):

Where
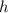 is the Planck constant and
is the Planck constant and
 is the speed of light in vacuum.
is the speed of light in vacuum.
On the other hand, it is known titanium metal requires a photon with a minimum energy
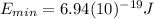 to emit electrons. This means, we need at least a wavelength
to emit electrons. This means, we need at least a wavelength
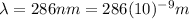 to fulfill this condition.
to fulfill this condition.
Therefore:
Since the wavelength range of visible light is between 400nm and 750nm, aproximately, and 286 nm is not in this range; it is impossible to to eject electrons from titanium metal using visible light.