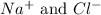Answer: The correct answer is option A.
Step-by-step explanation:
An ionic compound is defined as the compound formed by the complete transfer of electrons from one element (forming cation) to another element (forming anion)
Sodium (Na) is an element having atomic number 11 and its electronic configuration is
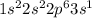
It will loose 1 electron to form
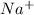 ion (cation)
ion (cation)
Chlorine (Cl) is an element having atomic number 17 and its electronic configuration is
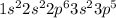
It will gain 1 electron to form
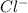 ion (anion)
ion (anion)
The ionic compound so formed will have the chemical formula of NaCl.
Hence, the correct answer is option A.