Answer:
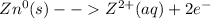
Step-by-step explanation:
Hello,
Considering the stated requirement, such half reaction indicates that pure zinc whose oxidation state is 0 becomes ionized zinc whose oxidation state is 2+; in such a way, its half reaction is:
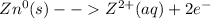
It is important to notice that 2 electrons were transferred since the change in the oxidation state is from 0 to +2:
Best regards.