Answer: The half reaction for the oxidation of solid gold is written below
Step-by-step explanation:
Oxidation reaction is defined as the reaction in which an atom looses its electrons. Here, oxidation state of the atom increases.
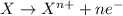
Reduction reaction is defined as the reaction in which an atom gains electrons. Here, the oxidation state of the atom decreases.
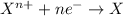
The half reaction for the oxidation of gold to gold (III) ions follows:
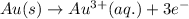
Hence, the half reaction for the oxidation of solid gold is written above.