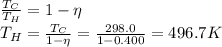Answer:
496.7 K
Step-by-step explanation:
The efficiency of a Carnot engine is given by the equation:
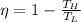
where:
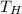 is the temperature of the hot reservoir
is the temperature of the hot reservoir
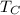 is the temperature of the cold reservoir
is the temperature of the cold reservoir
For the engine in the problem, we know that
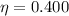 is the efficiency
is the efficiency
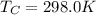 is the temperature of the cold reservoir
is the temperature of the cold reservoir
Solving for
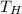 , we find:
, we find: