Answer:
New volume is 295.61 mL
Step-by-step explanation:
According Charles's law, at constant pressure the volume of a fixed mass of a gas and its absolute temperature are directly proportional.
Mathematically;
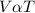
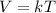
When the variables are varied then we have a constant equivalent to;
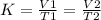
In this case, we are given;
Initial volume, V1 = 320.0 ML
Initial temperature, T1 = 55 °C or 328 K
New temperature, T2 = 30 °C or 303 K
We are required to calculate the new volume
This can be done by replacing the values of each variable in the equation;
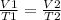
we get;
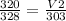
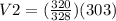
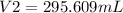
= 295.61 mL (2 d.p.)
Thus; the new volume is 295.61 mL