Answer:
The vapor pressure of ethanol in the solution is 10,27 kPa
Step-by-step explanation:
To obtain the vapor pressure of a solution it is necessary to use Raoult's law:
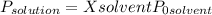 (1)
(1)
The moles of ethanol are:
18,00mL×
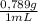 ×
×
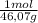 = 0,3083 mol Ethanol.
= 0,3083 mol Ethanol.
Moles of benzoic acid:
12,55 g×
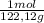 = 0,1028 mol benzoic acid.
= 0,1028 mol benzoic acid.
Thus, mole fraction of solvent, X, is:
 = 0,7499
= 0,7499
Replacing this value in (1):
 = 10,27 kPa
= 10,27 kPa
I hope it helps!