Answer:
3 × 10¹⁰⁹
Step-by-step explanation:
2Cr (s) → 2Cr³⁺ + 6e⁻
and,
Ec° for the above reaction = 0.74 V
also,
3Cu²⁺ (aq) + 6e⁻ → 3Cu(s)
and,
Ea° for the above reaction = 0.34 V
Now,
The cell potential, E° = 0.34 + 0.74 = 1.08
E° =
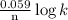
thus,
0.34 =
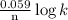
here, n is the number of electrons exchanged
n = 6
1.08 =
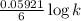
or
㏒ k = 109.44
taking anti-log both sides
we get
k = 3 × 10¹⁰⁹
Hence, the answer is option (A) 3 × 10¹⁰⁹