Step-by-step explanation:
It is given that mass of
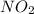 is 46.01 g/mol and mass of
is 46.01 g/mol and mass of
 is 63.01 g/mol.
is 63.01 g/mol.
So, in 1 mole calculate the number of moles present in 63.21 g/mol of
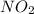 as follows.
as follows.
No. of moles =

=
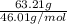
= 1.37 mol
Now, according to the reaction equation we require 3 moles of
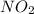 to make 2 moles of
to make 2 moles of
 .
.
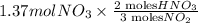
= 0.91 mol
Hence, calculate the mass of
 as follows.
as follows.
No. of moles =

0.91 mol =
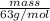
mass = 57.33 g
Now, mass of
 actually produced by 80.37% yield is calculated as follows.
actually produced by 80.37% yield is calculated as follows.
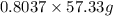
= 46.07 g
Thus, we can conclude that 46.07 g of nitric acid,
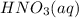 , are produced in the experiment.
, are produced in the experiment.