Answer:
Work transfer is - 97.02 KJ. It means that work is given to the system.
Heat transfer = - 97.02 KJ . It means that heat is rejected from the system.
Step-by-step explanation:
Given that
m= 3 kg
P₁=2 bar
T=T₁=T₂=30 °C
T=303 K
P₂=2.5 bar
PV= Constant
This is the isothermal process .
We know that work for isothermal process given as
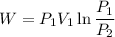
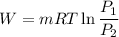
For air
R= 0.287 KJ/Kg.K
Now by putting the values
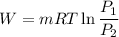
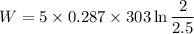
W= - 97.02 KJ
So the work transfer is - 97.02 KJ. It means that work is given to the system.
We know that for ideal gas internal energy is the only function of temperature.The change in internal energy ΔU
ΔU = m Cv ΔT
Here ΔT= 0
So
ΔU =0
From first law of thermodynamics
Q= ΔU +W
ΔU = 0
Q= W
Q= - 97.02 KJ
Heat transfer = - 97.02 KJ . It means that heat is rejected from the system.