Answer:
The method is accurate in the calculation of the
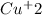
Step-by-step explanation:
As a first step we have to calculate the average concentration of
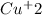 find it by the method.
find it by the method.
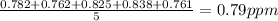
Then we have to find the standard deviation:
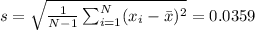
For the confidence interval we have to use the formula:
μ=Average±
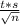
Where:
t=t student constant with 95 % of confidence and 5 data=2.78
μ=
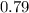 ±
±
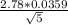
upper limit: 0.84
lower limit: 0.75
If we compare the limits of the value obtanied by the method (Figure 1 Red line) with the reference material (Figure 1 blue line) we can see that the values obtained by the method are within the values suggested by the reference material. So, it's method is accurate.