Answer:
The concentration of the Na₂S₂O₃ solution is 0,08037 M
Step-by-step explanation:
The reaction of the titration is:
2 Na₂S₂O₃(aq) + I₂(aq) → Na₂S₄O₆ +2 NaI(aq).
The moles of I₂ that react are:
0,02350L×
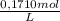 = 4,0185x10⁻³ moles of I₂
= 4,0185x10⁻³ moles of I₂
By the reaction of the titration, 1 mol of I₂ react with 2 moles of Na₂S₂O₃, the moles of Na₂S₂O₃ that react are:
4,0185x10⁻³ moles of I₂ ×
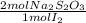 = 8,037x10⁻³ moles of Na₂S₂O₃
= 8,037x10⁻³ moles of Na₂S₂O₃
As the volume of the sample is 100,0mL≡0,1000L. The concentration of Na₂S₂O₃ is:
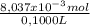 = 0,08037 M
= 0,08037 M
I hope it helps!