Step-by-step explanation:
It is given that a sample of a mixture of oxalic acid with water which has a wight of 0.2816 g.
This sample is titrated with 11.49 mL or 0.01149 L (as 1 ml = 0.001 L) of 0.0461 M NaOH.
Therefore, for complete neutralization, equate the total number of moles of
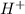 ions produced by
ions produced by
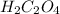 with total number of moles of
with total number of moles of
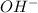 ions produced by NaOH.
ions produced by NaOH.
Assuming that weight of oxalic acid in the given sample is 'x'.
Hence, the total number of moles of
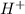 ions produced are as follows.
ions produced are as follows.
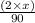 ........... (1) (We multiply by 2 because Oxalic Acid is a diprotic acid)
........... (1) (We multiply by 2 because Oxalic Acid is a diprotic acid)
So, number of moles of
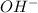 ions produced are as follows.
ions produced are as follows.
(0.0461 + 0.01149) = 0.05759 ............. (2)
Now, equating both we get the value of x as follows.
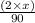 = 0.05759
= 0.05759
x = 2.591
Therefore, weight of oxalic acid in the sample is 2.591 g.
Percentage by mass of oxalic acid =
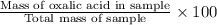
=
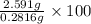
= 9.20%
Thus, we can conclude that mass by percent of oxalic acid in given sample of mass 0.2816 g is 9.20% .