Answer:
The pKa will be higher in hexane.
More H will dissociate in water because the dielectric constant is higher.
Step-by-step explanation:
In water, the ionisation of ionic compound increases because of higher value of dielectric constant of water. Higher value of dielectric constant of medium between two charges reduces the force between two charges. That is why ionisation of CH₃COOH will be higher in water.
In hexane the ionisation will be low so
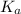 will be low , hence
will be low , hence
 will be high.
will be high.