Answer:
Diffusion time needed for 610 degree celcius is 81.91 min
Step-by-step explanation:
Given data:
Diffusion heat temperature = 826 + 273 = 1099 K
Diffusion time t_1 = 10 min
carbon concentration = 0.83%
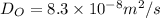
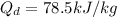
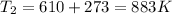
From Fick's formula we hvae
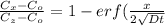
at specific concentration
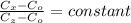
then
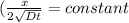
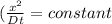
Dt = constant
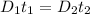
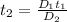
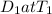
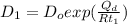
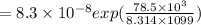
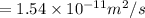
similarly for D_2 for T_2
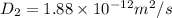
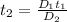
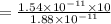
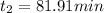
Diffusion time needed for 610 degree celcius is 81.91 min