Answer:
- A) pH = 2.42
- B) pH = 12.00
Step-by-step explanation:
The dissolution of HCl is HCl → H⁺ + Cl⁻
- To solve part A) we need to calculate the concentration of H⁺, to do that we need the moles of H⁺ and the volume.
The problem gives us V=2.5 L, and the moles can be calculated using the molecular weight of HCl, 36.46 g/mol:
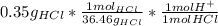 = 9.60*10⁻³ mol H⁺
= 9.60*10⁻³ mol H⁺
So the concentration of H⁺ is
[H⁺] = 9.60*10⁻³ mol / 2.5 L = 3.84 * 10⁻³ M
pH = -log [H⁺] = -log (3.84 * 10⁻³) = 2.42
- The dissolution of NaOH is NaOH → Na⁺ + OH⁻
- Now we calculate [OH⁻], we already know that V = 2.0 L, and a similar process is used to calculate the moles of OH⁻, keeping in mind the molecular weight of NaOH, 40 g/mol:
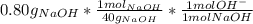 = 0.02 mol OH⁻
= 0.02 mol OH⁻
[OH⁻] = 0.02 mol / 2.0 L = 0.01
pOH = -log [OH⁻] = -log (0.01) = 2.00
With the pOH, we can calculate the pH:
pH + pOH = 14.00
pH + 2.00 = 14.00
pH = 12.00