Step-by-step explanation:
Hybridization of
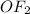 is
is
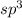
Bond pair in
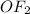 = 2
= 2
lone pair in
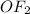 = 2
= 2
Because of presence of two lone pairs of electron shape is bent.
F is more electronegative than O, therefore two O-F bons are polar and hence, two dipole exists. The resultant diople moment in a molecule is the vector sum of individual existing dipoles (shown in the attachment).
because of the bent shape of
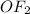 two dipole moments add to each other. this result in the existence of permanent dipole moment in the molecule and thus
two dipole moments add to each other. this result in the existence of permanent dipole moment in the molecule and thus
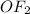 is a polar molecule.
is a polar molecule.