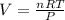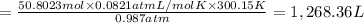Answer:
1,981.84 L of gas is formed from 1.40 lb of lithium hydride.
1,268.36 L of gas is formed from 1.40 lb of magnesium hydride.
Step-by-step explanation:
Hydrogen gas from lithium hydride:
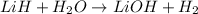
Mass of LiH = 1.40 lb = 635.0288 g
1 lb = 453.592 g
Moles of LiH =
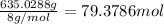
According to reaction,1 mole of LiH gives 1 mole of hydrogen gas.
Then 79.3786 mole of LiH will give :
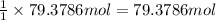 of hydrogen gas.
of hydrogen gas.
Volume of hydrogen gas = V
Pressure of hydrogen gas , P= 750 Torr =0.987 atm
1 Torr = 0.00131579 atm
Temperature of the gas = T = 27°C = 300.15 K
Moles of gas = n = 79.3786 moles

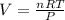
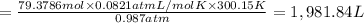
Hydrogen gas from magnesium hydride:
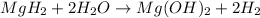
Mass of
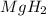 = 1.40 lb = 635.0288 g
= 1.40 lb = 635.0288 g
1 lb = 453.592 g
Moles of
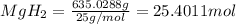
According to reaction,1 mole of
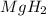 gives 2 mole of hydrogen gas.
gives 2 mole of hydrogen gas.
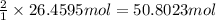 of hydrogen gas.
of hydrogen gas.
Volume of hydrogen gas = V
Pressure of hydrogen gas , P= 750 Torr =0.987 atm
1 Torr = 0.00131579 atm
Temperature of the gas = T = 27°C = 300.15 K
Moles of gas = n = 50.8023 moles