Answer:
The mass of hydrogen gas is collected is 0.073 grams.
Step-by-step explanation:
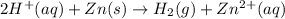
Volume of gas collected = V = 0.947 L
Temperature of the gas ,T= 25°C = 298.15 K
Total pressure of pressure of the gas = 743 mmHg
Vapor pressure of the water = 23.78 mmHg
Pressure of the gas = 743 mmHg - 23.78 mmHg =719.22 mmHg=0.9463 atm
1 atm = 760 mmHg
Moles of hydrogen gas = n
Using an ideal gas equation:

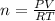
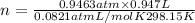
n = 0.03661 mol
Mass of 0.03661 moles of hydrogen gas = 0.03661 mol × 2 g/mol= 0.073 g