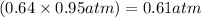Answer:
Partial pressure of Ne is 0.61 atm
Step-by-step explanation:
Let's assume mixture of He and Ne behaves ideally.
Then, according to Dalton's law for partial pressure in a mixture of gas-
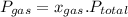
Where
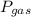 is partial pressure of a gas in mixture,
is partial pressure of a gas in mixture,
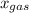 is mole fraction of a gas in mixture and
is mole fraction of a gas in mixture and
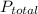 is total pressure of mixture.
is total pressure of mixture.
Here,
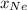 = (number of moles of Ne)/(Total number of moles in mixture)
= (number of moles of Ne)/(Total number of moles in mixture)
So,
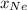 =
=
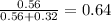
So,
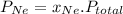 =
=