Answer:
The volume of CO₂ produced by this power plant is 3,466x10¹²L
Step-by-step explanation:
To obtain the volume of CO₂ produced you must use ideal gas law:
V = nRT/P
Where n are moles, R is gas constant (0,082atmL/molK), T is temperature and P is pressure (1,00atm)
The moles of CO₂ are:
6x10⁶ tons CO₂×
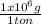 ×
×
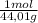 = 1,363x10¹¹ moles of CO₂
= 1,363x10¹¹ moles of CO₂
Temperature in Kelvin is:
37°C + 273,15 = 310,15 K
Thus, the volume of CO₂ produced by this power plant is:
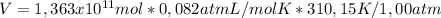
V = 3,466x10¹²L
I hope it helps!