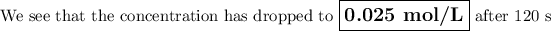Answer:
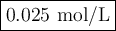
Step-by-step explanation:
The half-life (30.0 s) is the time it takes for half of the N₂O₅ to react.
After one half-life, half (50 %) of the original amount will remain.
After a second half-life, half of that amount (25 %) will remain, and so on.
We can construct a table as follows:
![\begin{array}{crcc}\textbf{No. of} && \textbf{Fraction} & \\\textbf{half-lives} & \textbf{t/s} & \textbf{remaining} &\rm \mathbf{{[N_(2)O_(5)] /(mol/L)}}\\0 & 0 & 1 & 0.400\\1 & 30.0 & 1/2 & 0.200\\2 & 60.0 & 1/4 & 0.100\\3 & 90.0 &1/8 & 0.050\\4 & 120.0 & 1/16 & 0.025\\5& 150.0 & 1/32 & 0.012\\\end{array}](https://img.qammunity.org/2020/formulas/chemistry/high-school/byrg3wq1jhnffpjwaw6ndhwoluve44adqg.png)