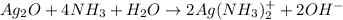Step-by-step explanation:
Tollens' reagent is prepared by using two-step process : -
Step 1:
Silver oxide is formed by mixing aqueous silver nitrate with base like sodium hydroxide. The reaction is shown below as:
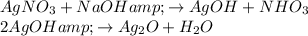
Step 2
Ammonia solution is drop-wise added until all the silver oxide dissolves to form the reagent. The reaction is shown below as: