Answer:
The ballon will brust at
Pmax = 518 Torr ≈ 0.687 Atm
Step-by-step explanation:
Hello!
To solve this problem we are going to use the ideal gass law
PV = nRT
Where n (number of moles) and R are constants (in the present case)
Therefore, we can relate to thermodynamic states with their respective pressure, volume and temperature.
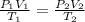 --- (*)
--- (*)
Our initial state is:
P1 = 754 torr
V1 = 3.1 L
T1 = 294 K
If we consider the final state at which the ballon will explode, then:
P2 = Pmax
V2 = Vmax
T2 = 273 K
We also know that the maximum surface area is: 1257 cm^2
If we consider a spherical ballon, we can obtain the maximum radius:

Rmax = 10.001 cm
Therefore, the max volume will be:
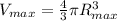
Vmax = 4 190.05 cm^3 = 4.19 L
Now, from (*)
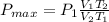
Therefore:
Pmax= P1 * (0.687)
That is:
Pmax = 518 Torr