Answer:
The correct answer is 12,48 J.
Step-by-step explanation:
According to the first law of thermodynamics, the variation of the internal energy is equal to the heat minus the work.
ΔU = Q - W
Being:
Q=n * C * (
 -
-
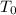 )
)
Q: Heat
n: number of moles
C: specific heat
 : Final temperature
: Final temperature
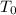 : Initial temperature
: Initial temperature
As in this situation there is no variation of the internal energy, the initial equation remains:
W=Q
First, we calculate the number of moles of Dysprosium present in a 10g sample.
n =
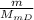
n: Number of moles of Dysprosium
m: Mass of Dysprosium
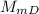 : Molar mass of Dysprosium
: Molar mass of Dysprosium
n =
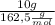
n=0,06 mol
Now, replacing the values given in the problem in the equation of Q, we calculate W as:
W = n * C * (
 -
-
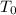 )
)
W=0,06 mol * 0,1733
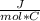 * (2600 C - 1400 C)
* (2600 C - 1400 C)
W=12,48 J
Have a nice day!