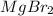Answer : The charge on magnesium is (+2) and the the charge on bromide is (-1).
Explanation :
Ionic compound : It is defined as the compound which is formed when electron gets transferred from one atom to another atom.
Ionic compound are usually formed when a metal reacts with a non-metal.
For formation of a neutral ionic compound, the charges on cation and anion must be balanced.
The cation is formed by loss of electrons by metals and anions are formed by gain of electrons by non metals.
Magnesium bromide is an ionic compound because magnesium element is a metal and bromide element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
The charge on magnesium is (+2) and the the charge on bromide is (-1). Both combine with the an ionic bond by the criss-cross method.
Thus, the formula of the compound magnesium bromide will be