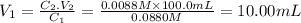Answer:
The volume of the original solution is 10.00 mL.
Step-by-step explanation:
We have a concentrated solution of C₁ = 0.0880 M and we want to prepare 100.0 mL (V₂) of a dilute one of C₂ = 0.0088 M. To calculate the volume of solution 1 (V₁) to be diluted we use the dilution rule.
C₁.V₁=C₂.V₂