Answer:
0.104 atm
Step-by-step explanation:
The equilibrium constant based on the partial pressure (Kp) depends only on the gas substances. For a generic reaction aA(g) + bB(g) ⇄ cC(g) + dD(g), it can be calculated by:
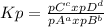 , where pX represents the partial pressure of X. So, for the reaction given, let's do a equilibrium table:
, where pX represents the partial pressure of X. So, for the reaction given, let's do a equilibrium table:
N₂(g) + O₂(g) ⇄ 2NO(g)
0.175 0.127 0 Initial
-x -x +2x Reacts (stoichiometry is 1:1:2)
0.175-x 0.127-x 2x Equilibrium
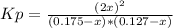
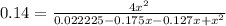
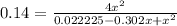
4x² = 0.0031115 - 0.04228x + 0.14x²
3.86x² + 0.04228x - 0.0031115 = 0
Using Bhaskara:
Δ = (0.04228)² - 4x3.86x(-0.0031115)
Δ = 0.04983
x = (-0.04228 +/ √0.04983)/(2*3.86)
x must be positive, so let's calculate only for the positive:
x = 0.0234 atm
So, the equilibrium partial pressure of oxygen is
0.127 - 0.0234 = 0.104 atm