Answer:
E= 3.497×10^(-19) J
Step-by-step explanation:
The energy gap between ground and the excited state will be equal to energy of a photon of wavelength 537 nm
which can be calculated as
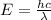
h= Planck's constant
λ= wavelength of photon
c= speed of light
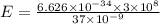
E= 3.497×10^(-19) J