Answer:
Mix the following to obtain the desired solution:
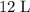 of the 100%-pure acid solution, and
of the 100%-pure acid solution, and
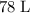 of the 22% acid solution.
of the 22% acid solution.
Assumption:
After mixing, the volume of the final solution is equal to the volume of the 22% solution plus the volume of the pure acid solution.
Explanation:
Let the volume of the pure acid required be
 liters.
liters.
The volume of the final solution is given to be 90 liters. If the assumption is true, the volume of the 22% solution has to be equal to
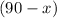 liters.
liters.
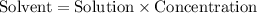 .
.
Amount of solvent from that
 liters pure acid solution:
liters pure acid solution:
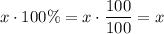 .
.
Amount of solvent from that
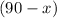 liters of 10% acid solution:
liters of 10% acid solution:
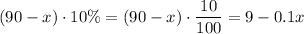 .
.
Solvent from the two solutions, combined:
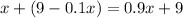 .
.
Concentration the mixed solution:
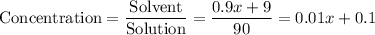 .
.
This concentration is expected to be equal to
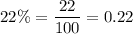 .
.
In other words,
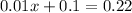 .
.
Solve for
 :
:
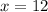 .
.
That is:
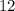 liters of the pure acid is required. Another
liters of the pure acid is required. Another
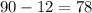 liters of the 22% solution will be required.
liters of the 22% solution will be required.