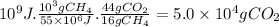Answer:
5.0 × 10⁴ g of CO₂
Step-by-step explanation:
First, we write the balanced equation for the combustion of methane.
CH₄ + 1.5 O₂ ⇄ CO₂ + H₂O
Now, we can establish the following relations:
- 16g of CH₄ produce 44g of CO₂
- 1 GJ = 10⁹ J
- 55 MJ (55 × 10⁶ J) are released for every 1 kg (10³g) of CH₄ burned.
Using proportions,