Answer:
19.27
Step-by-step explanation:
Some values are corrected from correct source.Thus,
Moles of SO₂ = 8.19x10⁻² moles
Moles of O₂ = 8.10x10⁻² moles
Volume = 1 L

Concentration of SO₂ = 8.19x10² M
Concentration of O₂ = 8.10x10 M
Considering the ICE table for the equilibrium as:
2SO₂ (g) + O₂ (g) ⇔ 2SO₃ (g)
t = o 8.19x10⁻² 8.19x10⁻²
t = eq -2x -x 2x
--------------------------------------------- --------------------------
neq: 8.19x10⁻² -2x 8.19x10⁻² -x 2x
Given:
Equilibrium concentration of O₂ = 5.98x10⁻² M = 8.19x10⁻² -x
Thus, x = 0.0212 M
[SO₂] = 8.19x10⁻² - 2*0.0212 = 0.0395 M
[SO₃] = 2*0.0212 = 0.0424 M
The expression for the equilibrium constant is:
![K_c=\frac {[SO_3]^2}{[SO_2]^2[O_2]}](https://img.qammunity.org/2020/formulas/chemistry/college/gwx14y3dsj4kidqa0zgosstz3wocr4m9i3.png)
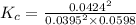
K = 19.27