Answer:
0,171 M NaCl and 0,168 m NaCl
Step-by-step explanation:
Molarity (M) is an unit of chemical concentration that relates moles of solute per liters of solution and molality (m) is another unit of chemical concentration that relates moles of solute per kg of solution.
For a solution of 10g/L of NaCl the molarity is:
10g/L NaCl×
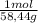 = 0,17 moles/L of NaCl≡ 0,171 M NaCl
= 0,17 moles/L of NaCl≡ 0,171 M NaCl
For molality you can know that in 1 L you have 0,17 moles of NaCl.
The kg of solution are:
0,010 kg of NaCl (solute) + 1 kg of solvent (Water, 1L of water is 1 kg) = 1,01 kg of solution.
Thus, molality of 10 g/L NaCl solution is:
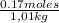 = 0,168 m NaCl
= 0,168 m NaCl
I hope it helps!