Answer:
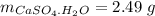
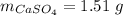
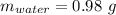
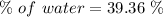
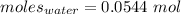
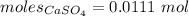
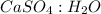 = 5 : 1
= 5 : 1
Step-by-step explanation:
Given :
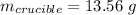
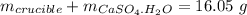
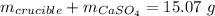
Mass of salt hydrate:
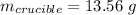
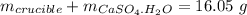
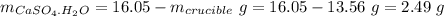
Mass of salt anhydrous:
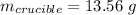
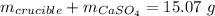
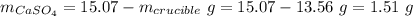
Mass of water:
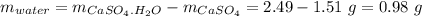
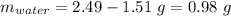
Percentage of water:
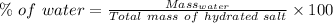
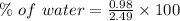
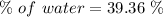
Moles of water:
Mass of water = 0.98 g
Molar mass of
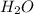 = 18 g/mol
= 18 g/mol
The formula for the calculation of moles is shown below:
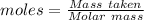
Thus, moles are:
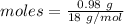
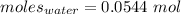
Moles of anhydrate salt:
Amount = 1.51 g
Molar mass of
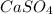 = 136.14 g/mol
= 136.14 g/mol
The formula for the calculation of moles is shown below:
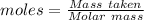
Thus, moles are:
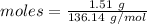
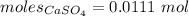
The simplest ration of the two are:
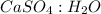 = 0.0111 : 0.0544 = 5 : 1
= 0.0111 : 0.0544 = 5 : 1