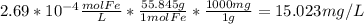Answer:
1.345*10⁻⁴ mol/L
15.023 mg/L
Step-by-step explanation:
The chemical formula of ferrocene is Fe(C₅H₅)₂, thus its molecular weight is:
55.845 g/mol + 10*12g/mol + 10 *1g/mol = 185.845 g/mol
- The moles of Fe contained in 25 mg (or 0.025 g) of ferrocene are:
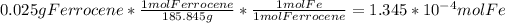
- The final volume is 500 mL, or 0.5 L. So the iron concentration in mol/L is:
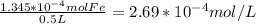
- We can convert that value into mg/L: