Answer:
For 1: The correct answer is Option A.
For 2: The correct answer is Option C.
For 3: The correct answer is Option B.
For 4: The correct answer is Option B.
Step-by-step explanation:
We are given:


As, enthalpy of the reaction is negative. So, it is an exothermic reaction.
For an exothermic reaction, heat is released during a chemical reaction and is written on the product side.
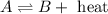
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle. This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
As, heat is released during a chemical reaction. This means that temperature is decreased on the reactant side. If the temperature in the equilibrium is decreased, the equilibrium will shift in the direction where, temperature is getting increased. Thus, the reaction will shift in right direction that is towards the product.
There are 3 conditions:
- When
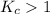 ; the reaction is product favored.
; the reaction is product favored. - When
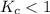 ; the reaction is reactant favored.
; the reaction is reactant favored. - When
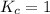 ; the reaction is in equilibrium.
; the reaction is in equilibrium.
On decreasing the temperature of the system, the equilibrium is shifting to right direction. This means that more and more product is getting formed which increases the value of

Hence, the correct answer is Option A.
 is the constant of a certain reaction at equilibrium while Q is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.
is the constant of a certain reaction at equilibrium while Q is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.
There are 3 conditions:
- When
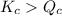 ; the reaction is product favored.
; the reaction is product favored. - When
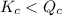 ; the reaction is reactant favored.
; the reaction is reactant favored. - When
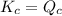 ; the reaction is in equilibrium.
; the reaction is in equilibrium.
As, the value of
 is getting increased. This means that value of
is getting increased. This means that value of
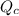 is less than the equilibrium constant.
is less than the equilibrium constant.
Hence, the correct answer is Option C.
As, the reaction is proceeding the forward direction. So, to re-establish equilibrium, the reaction must proceed in the reverse (backward) direction to counter the effect.
Hence, the correct answer is Option B.
For the given chemical reaction:
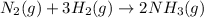
Hydrogen gas is present on the reactant side and the reaction is going in the forward direction.
This means that more and more product is getting formed. So, the concentration of reactants will decrease.
Hence, the correct answer is Option B.