Step-by-step explanation:
It is given that mass of acetic acid is 17.9 g and freezing point is given as
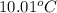 .
.
For acetic acid,
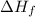 = 0.185273 kJ/g
= 0.185273 kJ/g
Therefore, total heat removed to convert 17.9 g of acetic acid from liquid to solid is as follows.
Q =
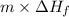
=
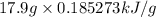
= 3.316386 kJ
Thus, we can conclude that 3.316386 kJ energy must be removed to convert all 17.9 g of liquid acetic acid to solid acetic acid.