Answer:
a) Partial pressure of benzyl alcohol is 0,23 atm
b) Fraction of benzyl alcohol dissociated at equilibrium is 0,99
Step-by-step explanation:
The dissociation of benzyl alcohol is:
C₆H₅CH₂OH(g) ⇄ C₆H₅CHO(g) + H₂(g)
With ke = [C₆H₅CHO] [H₂] / [C₆H₅CH₂OH]
The molarity of benzyl alcohol is:
11,56g×
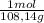 = 0,1069 moles of benzyl alcohol/19,85L = 5,385x10⁻³M
= 0,1069 moles of benzyl alcohol/19,85L = 5,385x10⁻³M
The concentrations in equilibrium after the addition of benzyl alcohol are:
[C₆H₅CH₂OH] = 5,385x10⁻³M - x
[C₆H₅CHO] = x
[H₂] = x
Where x are the moles produced of both benzaldehyde and hydrogen chemicals.
Replacing:
0,558 = [C₆H₅CHO] [H₂] / [C₆H₅CH₂OH]
0,558 = [x] [x] / [5,385x10⁻³-x]
x² + 0,558x - 3x10⁻³
Solving:
x = -0,5633No chemical sense. There are not negative concentrations
x = 0,00533 Real answer.
a) Molarity of benzaldehyde is 0,00533
By ideal gas law:
P = M RT
Where P is pressure
M is molarity (0,00533M)
R is gas constant (0,082atmL/molK)
T is temperature (250+273,15 = 523,15K)
Replacing, partial pressure of benzyl alcohol is P = 0,23 atm
b) The benzyl alcohol dissociated is 0,00533M. The initial molarity of benzyl alcohol is 5,385x10⁻³M. Thus, fraction of benzyl alcohol dissociated at equilibrium is 0,00533/5,385x10⁻³ = 0,99
I hope it helps!