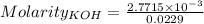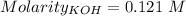Answer:
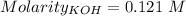
Step-by-step explanation:
Moles of KHP :
Given, Mass of KHP = 0.566 g
Molar mass of KHP = 204.22 g/mol
The formula for the calculation of moles is shown below:

Thus,
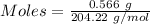
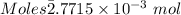
According to the reaction shown below:
KHP + KOH ⇒ KKP + H₂O
1 mole of KHP reacts with 1 mole of KOH
So,
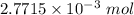 of KHP reacts with
of KHP reacts with
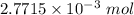 of KOH
of KOH
Moles of KOH =
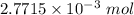
Volume = 22.9 mL = 0.0229 L ( 1 mL = 0.001 L)