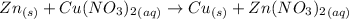Answer:
No reaction
Step-by-step explanation:
Single-displacement reaction is the chemical reaction in which one metal which has higher negative redox potential replaces the another from it's salt solution.
Thus, Cu cannot replaces zine from its salt as it is less reactive than zince.
However, zinc can replace copper from its salt as: