Step-by-step explanation:
When iodine heptafluoride reacts rapidly with water to give a mixture of periodic acid and hydrofluoric acid. Reaction equation for the same is as follows.
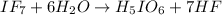
As it is given that there are
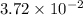 moles of iodine heptafluoride are present. Molar mass of
moles of iodine heptafluoride are present. Molar mass of
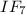 is 259.9 g/mol. Molar mass of
is 259.9 g/mol. Molar mass of
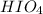 is 227.94 g/mol and molar mass of HF is 20.01 g/mol.
is 227.94 g/mol and molar mass of HF is 20.01 g/mol.
Now, according to the reaction equation
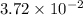 M
M
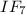 gives
gives
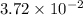 M
M
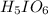 .
.
Also, the volume is given as 795 ml or 0.795 L (as 1 mL = 0.001 L).
Hence, calculate the concentration of
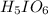 into the solution as follows.
into the solution as follows.
Concentration =
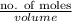
=
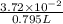
=
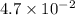 mol/L
mol/L
or, = 0.047 M
Now, as 1 mol of
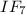 produces 7 mol HF. So,
produces 7 mol HF. So,
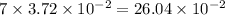 M HF.
M HF.
Therefore, concentration of HF will be calculated as follows.
Concentration of HF =
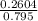
= 0.33 M
Thus, we can conclude that concentration of periodic acid is 0.047 M and concentration of hydrofluoric acid is 0.33 M.