Answer: Option (A) is the correct answer.
Step-by-step explanation:
A neutralization reaction is defined as the reaction in which an acid reacts with a base to yield salt and water.
The given reaction will be as follows.

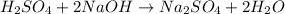
Now, if molarity of NaOH =
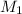 and volume of NaOH =
and volume of NaOH =

Therefore, Molarity of HCl = molarity of
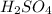 =
=
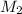
Volume of HCl required =
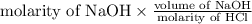
=
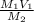
So, vVolume of
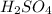 required = 0.5 x molarity of NaOH x \frac{\text{volume of NaOH}}{\text{molarity of H_{2}SO_{4}}}[/tex]
required = 0.5 x molarity of NaOH x \frac{\text{volume of NaOH}}{\text{molarity of H_{2}SO_{4}}}[/tex]
=
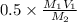
Hence, volume of HCl = 2 x volume of
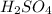 .
.
Thus, we can conclude that HCl solution would be required more volume (in mL) to neutralize the base.