Answer:
The percent ionization is 0,66%.
Step-by-step explanation:
K for acetic acid = 1,74x10*-5
CH3COOH (ac) + H2O(l) ⇄ H3O+ (ac) + CH3COO- (ac)
initial. 0,40 M ---- ------
react. X X X
eq. (0,4 -X) X X
This is the expression for K:
Ka = ( [H3O+] . [CH3COO-] ) / [CH3COOH]
1,74x10*-5 = (X . X) / 0,40 - X
Since the X in the numerator is going to be a very small number, since my Ka is in the order of -5, I can despise it in order to avoid the quadratic equation. You can't always do that!.. Be careful and pay attention to the value of Ka.
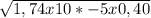 = X
= X
X = 2,64x10*-3
% ionization is (X /acid concentration) x 100
(2,64x10*-3 / 0,4) x 100 = 0,66%