Answer:
18,1 mL of a 0,304M HCl solution.
Step-by-step explanation:
The neutralization reaction of Ba(OH)₂ with HCl is:
2 HCl + Ba(OH)₂ → BaCl₂ + 2 H₂O
The moles of 17,1 mL≡0,0171L of a 0,161M Ba(OH)₂ solution are:
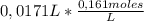 = 2,7531x10⁻³moles of Ba(OH)₂
= 2,7531x10⁻³moles of Ba(OH)₂
By the neutralization reaction you can see that 2 moles of HCl reacts with 1 mole of Ba(OH)₂. For a complete reaction of 2,7531x10⁻³moles of Ba(OH)₂ you need:
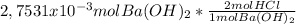 = 5,5062x10⁻³moles of HCl.
= 5,5062x10⁻³moles of HCl.
The volume of a 0,304M HCl solution for a complete neutralization is:
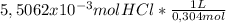 = 0,0181L≡18,1mL
= 0,0181L≡18,1mL
I hope it helps!