Answer:
option (A) Glass
Step-by-step explanation:
Given:
Specific heat of water = 4.184 J/g°C
Specific heat of glass = 0.780 J/g°C
Amount of heat added, Q = 10 J
Mass of glass = 1.00 g
Mass of water = 1.00 g
Now,
The heat provided:
Q = mcΔT
here,
m is the mass
c is the specific heat
ΔT is the change in the temperature
or rearranging, we get
ΔT =
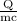
therefore,
the change in temperature is directly proportional heat added and inversely proportional to the mass and the specific heat
here,
except specific heat capacity all the values are equal
therefore,
the substance with lower specific heat will experience the larger increase of temperature.
Hence, the glass will experience the larger increase of temperature as its specific heat is lower
so the correct answer is option (A) Glass