Answer:
197mL of 0,506M HCl
Step-by-step explanation:
The reaction of HCl + BaCO₃ is:
BaCO₃(s) + 2HCl → BaCl₂(aq) + CO₂ + H₂O.
The moles of BaCO₃ in 9,85 g are:
9,85 g of BaCO₃ ×
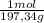 = 0,0499 moles of BaCO₃
= 0,0499 moles of BaCO₃
As 1 mol of BaCO₃ reacts with two moles of HCl, for a complete reaction of BaCO₃ to dissolve this compound in water you need:
0,0499 moles of BaCO₃ ×
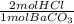 = 0,0998 moles of HCl
= 0,0998 moles of HCl
If you have a 0,506M HCl, you need to add:
0,0998 moles of HCl×
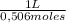 = 0,197 L ≡ 197mL
= 0,197 L ≡ 197mL
I hope it helps!