Step-by-step explanation:
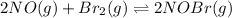
Equilibrium constant of reaction =
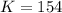
Concentration of NO =
![[NO]=(2.69* 10^(-2) mol)/(1 L)=2.69* 10^(-2) M](https://img.qammunity.org/2020/formulas/chemistry/college/d77da6wnz1g9zw6snsdele7khx9nlfiylf.png)
Concentration of bromine gas =
![[Br_2]=(3.85* 10^(-2) mol)/(1 L)=3.85* 10^(-2) M](https://img.qammunity.org/2020/formulas/chemistry/college/fj0bisirk5hgucvxurqntt0bwzg2i3k3mg.png)
Concentration of NOBr gas =
![[Br_2]=(9.56* 10^(-2) mol)/(1 L)=9.56* 10^(-2) M](https://img.qammunity.org/2020/formulas/chemistry/college/s0pdlzryjmn4tzdhfjmls7z9rib4iio2cw.png)
The reaction quotient is given as:
![Q=([NOBr]^2)/([NO]^2[Br_2])=((9.56* 10^(-2) M)^2)/((2.69* 10^(-2) M)^2* 3.85* 10^(-2) M)](https://img.qammunity.org/2020/formulas/chemistry/college/1pbuvar5qezs4lu0p6bvcwklf2pjgyacsi.png)
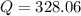
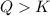
The reaction will go in backward direction in order to achieve an equilibrium state.
1. In order to reach equilibrium NOBr (g) must be produced. False
2. In order to reach equilibrium K must decrease. False
3. In order to reach equilibrium NO must be produced. True
4. Q. is less than K . False
5. The reaction is at equilibrium. No further reaction will occur. False