Answer:
Molarity of the solution? 0,262 M.
Concentration of the potassium cation? 0,786 M.
Concentration of the phosphate anion? 0,262 M.
Step-by-step explanation:
Potassium phosphate (K₃PO₄; 212,27 g/mol) dissolves in water thus:
K₃PO₄ → 3 K⁺ + PO₄³⁻ (1)
Molarity is an unit of chemical concentration given in moles of solute (K₃PO₄) per liters of solution.
There are 250 mL of solution≡0,25 L
The moles of K₃PO₄ are:
13,9 g of K₃PO₄ ×
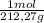 = 0,0655 moles of K₃PO₄
= 0,0655 moles of K₃PO₄
The molarity of the solution is:
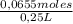 = 0,262 M
= 0,262 M
In (1) you can see that 1 mole of K₃PO₄ produces 3 moles of potassium cation. The moles of potassium cation are:
0,0655 moles×3 = 0,1965 moles
The concentration is:
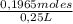 = 0,786 M
= 0,786 M
The moles of K₃PO₄ are the same than moles of PO₄³⁻, thus, concentration of phosphate anion is the same than concentration of K₃PO₄. 0,262 M
I hope it helps!